Retirement and forum shutdown (17 Jan 2022)
Hi,
John Howell who has managed the forum for years is getting on and wishes to retire from the role of managing it.
Over the years, he has managed the forum through good days and bad days and he has always been fair.
He has managed to bring his passion for fish keeping to the forum and keep it going for so long.
I wish to thank John for his hard work in keeping the forum going.
With John wishing to "retire" from the role of managing the forum and the forum receiving very little traffic, I think we must agree that forum has come to a natural conclusion and it's time to put it to rest.
I am proposing that the forum be made read-only from March 2022 onwards and that no new users or content be created. The website is still registered for several more years, so the content will still be accessible but no new topics or replies will be allowed.
If there is interest from the ITFS or other fish keeping clubs, we may redirect traffic to them or to a Facebook group but will not actively manage it.
I'd like to thank everyone over the years who helped with forum, posted a reply, started a new topic, ask a question and helped a newbie in fish keeping. And thank you to the sponsors who helped us along the away. Hopefully it made the hobby stronger.
I'd especially like to thank John Howell and Valerie Rousseau for all of their contributions, without them the forum would have never been has successful.
Thank you
Darragh Sherwin
Kalkwasser setup
- Seany (Sean Phelan)
- Offline
- Junior Member
-

- Posts: 224
- Thank you received: 0
Just thought I'd share my latest addition to my reef tank. With almost 40 coral pieces now insitu my Calcium and Alkalinity were suffering from reduced levels as the corals were using them at great rate as they grew. Calcium was hovering around 380mg/L and Alk about 6 dKh (2.14 meq/L) I was adding two part calcium / Alk supplements but they are expensive and levels were see-sawing up and down. My other problem that evaporation from the tank amounted to some 5 - 8 litres per day which also meant see-sawing salinity measurements as I nightly added RO water to replace that lost to evaporation.
The obvious choice was a calcium reactor but I didn't want to go down this route until I upgrade to a larger tank when I move house next year. Costly too!
The addition of Lime Water (Kalkwasser) was next considered. However I didn't want to be making up Limewater everynight, waiting for it to dissolve, mix with RO water, and then top up the sump. I also looked into an auto top-up system to reduce nightly addition of RO Water in one go.
Two items were purchased,
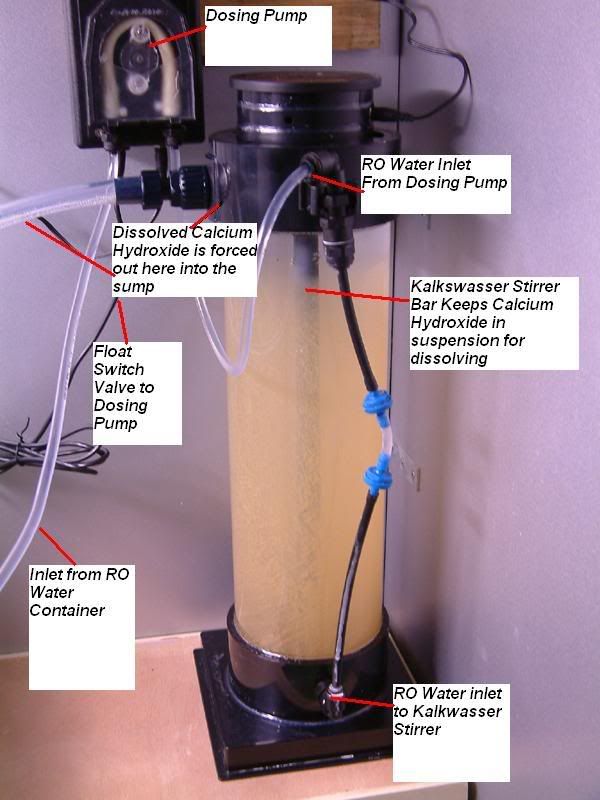
1. Aquamedic Niveaumat Dosing pump with float switch
1. Aquamedic Kalkwasser stirrer KS1000
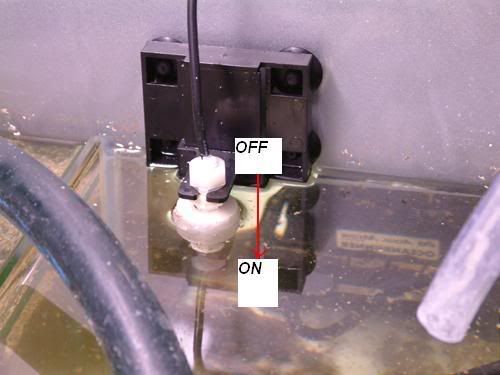
Photo from the right hand side of the sump area. When float switch drops due to evaporation, a signal is sent to the dosing pump to turn on.
The dosing pump pulls RO water from the 25litre Ro container and forces it into the bottom of the Kalkwasser stirrer.
The Kalkwasser stirrer has a central stirring bar that slowly agitates the calcium hydroxide powder that has settled at the bottom of the device. Fresh RO water enter the stirrer at the bottom and forces the supersaturated clear Liquid at the top out of the stirrer and into the sump. From here it is pumped up to the main display tank.
Alot of evaporation in this fine weather!!
In the tank water the hydroxide reacts with dissolved carbon dioxide to form bicarbonate (OH+CO2 = HCO3)
An important reason why I linked the Kalkwasser stirrer to an auto top up system, is that if I was to manually dose Lime Water each evening. The quick addition of so much hydroxide would react with bicarbonate forming calcium carbonate, which is unsoluble. Thus achieving the opposite of what I wanted. Also. Lime water has a pH of 12.4, which would have major health risks to the reef if added too quickly.
In summary,
A. All evaporated water is now automatically topped up as needed meaning less manual intervention and greater stability in salinity/SG for the inhabitants.
B. By feeding the RO water through a Kalkwasser stirrer, I get to automatically keep my Calcium and Alk levels at the optimum level.
C. The Calcium Hydroxide power will only need to be replenished about every 2 weeks.
D. Calcium / Alk will always be at optimum levels for the Corals to utilise. thus not check in their growth.
E. pH levels will also be kept at optimum levels due to the pH of the hydroxide
F. An added benefit of Kalkwasser is that phosphate is precipitated out of solution into hydroxylapatite. No Phosphate - no unwanted algae growths.
Current thinking in the reef world is that for reef aquaria with high calcium / Alk requirement a combined approach is required, namely a calcium reactor with a Kalkwasser stirrer as in syatems with low evaporation or during cool weather a Kalkwasser stirrer will not be adding enough calcium hydroxide to meet demand. Also the calcium reactor produces free CO2 that can be utilised by the Kalkwasser. Also phosphates which are a by product of the calcium reactor as it dissolves its media is harmlessly precipated by the Kalkwasser.
My setup was done 1 week ago and the results speak for themselves
Thursday 1st May
Ph 8.0
Calcium 380mg/L
Alk 6dKh
Wednesday 7th May
Ph 8.6
Calcium 440mg/L
Alk 9dKh
Hope you enjoyed my little informative article. Any questions, drop us a line.
Kind regards
Seany
Please Log in to join the conversation.
- Pablo (Pablo -)
- Offline
- Junior Member
-

- Posts: 176
- Thank you received: 0
Please Log in to join the conversation.
- Glenn 10 (glenn hornibrook)
- Offline
- Junior Member
-

- Posts: 14
- Thank you received: 0
Please Log in to join the conversation.
- Seany (Sean Phelan)
- Offline
- Junior Member
-

- Posts: 224
- Thank you received: 0
Sorry for not getting back to you sooner, but I was away for a few days.
From your parameters, I would say that your alkalinity and pH are fine. but your calcium is too low at 320mg/L. In order for kalkwasser to work effectively there must be sufficient evaporation from your aquarium to enable adequate dosing of saturated kalkwasser to maintain calcium levels. Otherwise you might have to look at alternative ways to maintain calcium levels i.e regular water changes introduces calcium in the new salt mix or one of the two part additive to manually dose calcium / alk. Failing that a calcium reactor (expensive) is your only man.
What is the total system volume of your tank and what is the daily evaporation rate?
Another question I would ask is how long are you dosing kalkwasser? The reason I ask this is if recently implemented, the kalkwasser is trying to bring your levels up from a historically very low level which you mentioned was 260mg/L. It is probably still on the way up given time and enough evaporation. If you have been using kalkwasser for some time, something I could recommend is that you use one of the 2 part solutions to slowly bring up your calcium levels closer to 420-450mg/L over a couple of days and then see if the kalkwasser is able to maintain these levels for you. If not, you evaporation rate is probably too low.
Magnesium is another factor to consider. Magnesium levels are usually stable and are easily maintained with routine water changes. However, if calcium precipitation occurs, or the hobbyist does not do regular water changes, magnesium levels can decline. The recommended level of magnesium is between 1300-1500 ppm. If magnesium levels do fall, calcium will be much more difficult to get to the proper levels of concentration. What are your mag levels like?
do you think it would be a problem to have the lime water extracted from a more milkey part of the solution.?
To answer your question, the problem with that I see is that by using the clear solution you are ensuring that the water being used for top-up for evaporation is a supersaturated solution of calcium hydroxide ensuring maintenace of both calcium and alkalinity in the tank.
If you added the milky solution i.e. unsaturated calcium hydroxide you risk the reaction of of excess calcium hydroxide with co2 causing precipation of calcium carbonate leading to you actually lowering the calcium levels in the aquarium:ohmy: . Calcium carbonate will not redissolve unless exposed to a very low pH. It has also been hypothesized (Delbeek & Sprung)that these fine particles of calcium carbonate may act as a \"seed\" site for ctystallization of more calcium carbonate within the aquarium itself and thus lowering alkalinity:ohmy: .
So you see if you use the milky part of the solution you risk achieving the opposite of what you are trying to aim for, namely increase calcium and alkalinity.
Can I recommend the following article as an excellent introduction to the mysteries of calcium management in a reef tank.
www.aquariacentral.com/articles/calcium.shtml
Hope this answers your question?
Kind regards
Seany
PS some more details of your setup and a few pics would be great to see!!
Please Log in to join the conversation.
- platty252 (Darren Dalton)
-

- Offline
- Moderator
-

- Posts: 2309
- Thank you received: 127
Please Log in to join the conversation.